Chemists Help Discover Mechanism Behind Important Biosynthetic Reaction
Carnegie Mellon University chemists have helped discover the reaction mechanism behind the biosynthesis of kainic acid, an important natural product used to study neurodegenerative disorders.
First harvested from the red seaweed Digenea simplex nearly 70 years ago, kainic acid was originally used to treat intestinal worm infections in Japan, said Yisong (Alex) Guo, associate professor of chemistry and a co-author of this study. However, the compound was later discovered to be a potent neuroexcitant that activates glutamate, the primary neurotransmitter in the human nervous system. Nowadays, kainic acid is frequently used by neuroscientists to excite certain regions of model organisms’ brains in research that aims to better understand neurological conditions.
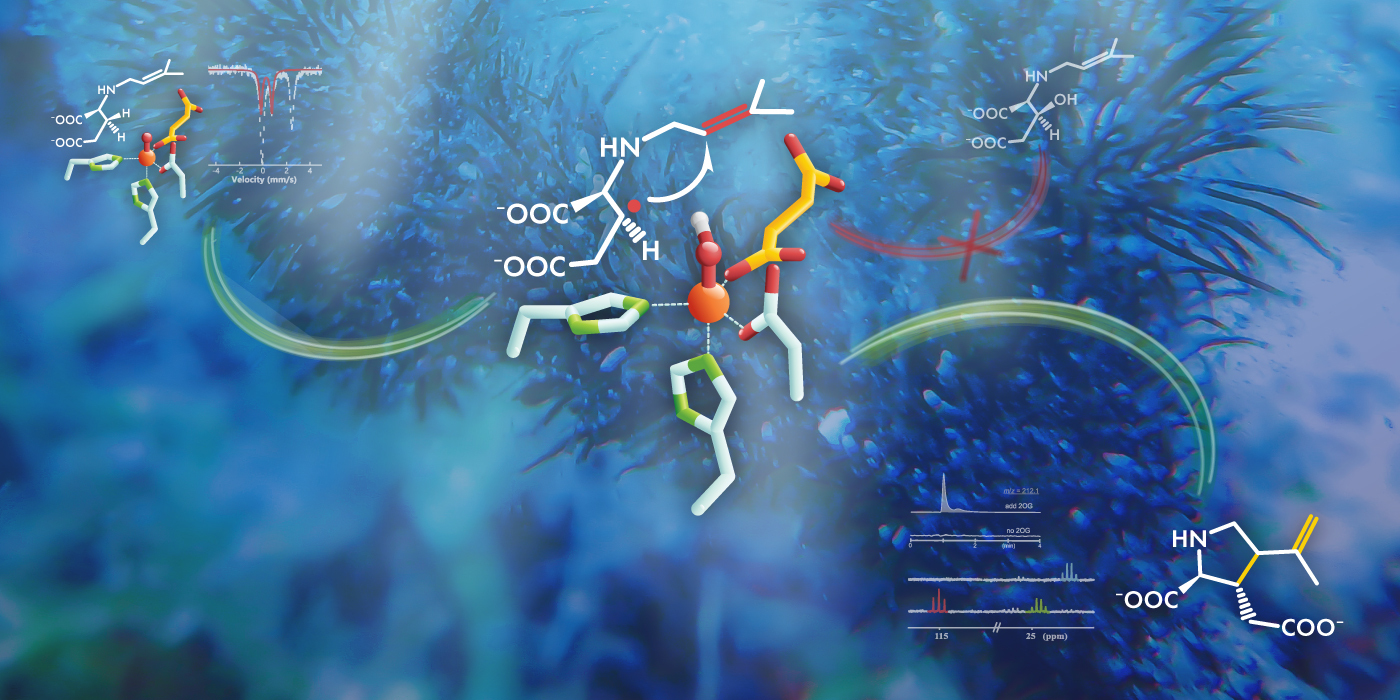
In recent years, however, the difficulty of harvesting natural kainic acid has made the product prohibitively expensive for many researchers to use, and scientists have not had much luck producing synthetic versions of it either. “Although there are more than 70 synthetic routes to make kainic acid, these methods are generally lengthy and complex, which make a large-scale synthesis difficult,” Guo said. In close collaboration with researchers from North Carolina State University led by Wei-chen Chang, Guo has been working to shed light on the mechanism of kainic acid biosynthesis catalyzed by the iron-containing enzyme KabC in hopes of making the production of the substance easier, and to better understand how metalloenzymes catalyze oxidative cyclization reactions and carbon–carbon bond formation reactions.
The researchers deployed an array of experimental techniques, including molecular probe synthesis and various forms of spectroscopy, targeted at the final step of the biosynthesis, which consists of an oxidative cyclization reaction via intramolecular carbon-carbon bond formation. “We elucidated for the first time that the intramolecular carbon-carbon bond formation is initiated by a carbon–hydrogen bond activation on one side of the KabC substrate,” Guo said. Then, a subsequently formed carbon radical attacks a carbon–carbon double bond on the other side of the substrate to form a new carbon–carbon bond, completing the cyclization step. The overall reaction finally finishes through another carbocation-triggered desaturation, and kainic acid is created.
“We are working on other carbon–carbon bond formation enzymes to understand whether the catalytic strategy utilized in KabC is one of the universal ways to install carbon–carbon bonds in enzymatic reactions,” Guo said of the next steps for this research. In particular, he and his collaborators are working to understand what controls the options of enzymatic reactions, since many other enzymes like KabC lead to different outcomes than what was seen in this biosynthesis.
“We will try to unravel the molecular governing factors that determine the selectivity in KabC,” Guo said. “This could help harness the power of enzymatic reactions to create new biocatalysts in order to access synthetically challenging reactions.”
Chemists Help Shed Light on How Iodine-Containing Molecules Contribute to the Formation of Atmospheric Aerosols
As part of a worldwide collaboration, Carnegie Mellon chemists have helped discover that iodic acids can rapidly form aerosol particles in the atmosphere, giving scientists more knowledge of how iodine emissions can contribute to cloud formation and climate change.
“Essentially all uncertainty around climate change and the atmosphere has something to do with particles and cloud droplets,” said Neil Donahue, Thomas Lord University Professor of Chemistry and a professor in the departments of Chemical Engineering and Engineering and Public Policy. The Donahue lab has been a longtime member of the CERN CLOUD experiment, an international collaboration of scientists that uses a special chamber at CERN in Switzerland to remedy that uncertainty by studying how cosmic rays affect the formation of particles and clouds in the atmosphere. The chamber allows researchers to precisely mix vaporous compounds and observe how particles form and grow from them.
In a study published in the journal Science, the CLOUD collaboration looked in particular at how vapors containing iodine affect this nucleation process. For reasons not yet fully understood, Donahue said, concentrations of iodine-containing vapor compounds have been increasing in recent years in the atmosphere.
“This represents a new pathway for particle formation, which in turn governs the properties of clouds in the marine atmosphere,” Donahue said.
Building on previous research his lab conducted on discovering a new rapid mechanism for atmospheric particle formation from nitric acid and ammonia vapors, Donahue and his team have now helped the CLOUD collaboration discover that the nucleation rates of iodic acid particles are very fast. This means that increasing concentrations of iodine-containing vapors in the atmosphere can lead to large increases in the number of particles that form clouds.
Specifically, Donahue and his collaborators, including current Ph.D. candidates Mingyi Wang and Victoria Hofbauer, alumna Qing Ye and former postdoctoral scholar Dexian Chen, contributed their use of a state-of-the-art chemical ionization mass spectrometer that can measure the amount and composition of extremely small particles less than 10 nanometers in size just following their formation.
“The CMU measurements showed that the newly formed particles are composed largely of iodic acid, confirming that this critical molecule not only is present as a vapor while particles are forming but definitively drives their growth,” Donahue said.
While clouds forming may sound like a relatively benign outcome, clouds play an important role in regulating Earth’s temperature because they are highly reflective. Much of the sun’s energy is reflected by clouds back into space, keeping Earth from becoming too hot. However, that reflectivity can work both ways, which is a particular problem at Earth’s poles. Typically, the white snow and ice surfaces reflect a lot of sunlight back into space, thus keeping the surface there cool. However, increased cloud formation in those regions can mean that the light reflected off the surface can be reflected back onto the ice and snow by the cloud cover.
“The Arctic is an especially vulnerable region, with twice the rate of warming and the huge consequences of both sea ice and ice sheet melting,” Donahue said. He and his lab are already planning future research into the complex feedbacks between iodic acid and sulfur compounds and how these affect the polar atmosphere and climate change.
“We have a great deal more to learn in this area, especially regarding the interactions of the iodine compounds and particles and dimethyl sulfide oxidation and its particle formation,” Donahue said.
Wildfires May Have Larger Effects on Cloud Formation and Climate Change than Previously Thought
As the frequency and size of wildfires continues to increase worldwide, research from Carnegie Mellon University scientists shows how the chemical aging of the particles emitted by these fires can lead to more extensive cloud formation and intense storm development in the atmosphere. The research was published in the journal Science Advances.
“The introduction of large amounts of ice-nucleating particles from these fires can cause substantial impacts on the microphysics of clouds, whether supercooled cloud droplets freeze or remain liquid, and the propensity of the clouds to precipitate,” said Ryan Sullivan, associate professor of chemistry and mechanical engineering. Understanding these impacts is a key factor in accurately modeling Earth’s climate and how it could continue to change.
In particular, the team was interested in ice nucleating particles, rare types of particles that can catalyze ice crystal formation in the atmosphere at higher than usual temperatures and thus greatly affect climatic processes. In fact, most precipitation over land starts from ice-containing clouds.
While it was already widely known that particles freshly emitted by the burning of biomass can impact ice nucleation greatly, Sullivan’s team was interested in finding out the effects of these particles as they traveled in the atmosphere and experienced chemical aging. The researchers analyzed the particles emitted by the burning of various types of plant material and simulated the aging processes these particles would undergo.
Typically, ice nucleating particles lose their potency as they age in the atmosphere, but the researchers found that the ice nucleating ability of particles emitted by burning biomass actually increased as they experienced simulated atmospheric aging. This represents a very different framework for considering how the climate-forcing properties of a major episodic source of particles evolve in the atmosphere.
The data from this study could have a major impact on future research into wildfires and climate change, said Lydia Jahl, who recently received her Ph.D. in chemistry from Carnegie Mellon in Sullivan’s group.
“We estimated that burning just one square meter of grassland could influence the concentration of ice nucleating particles in hundreds of thousands of cubic kilometers of the atmosphere,” Jahl said. “Climate modelers could use our data further to determine how wildfire emissions influence the balance of incoming solar radiation and outgoing terrestrial radiation, among other cloud properties.”