Faculty Notes
East Palestine train derailment
lends lessons for courses
Images of a huge plume of black smoke rising above derailed train cars in East Palestine, Ohio flooded Carnegie Mellon University students’ news feeds in early February. With East Palestine only about 50 miles northwest of Pittsburgh, students were uneasy and looked to their chemistry professors for reassurance.
“I told my students that my main concern is in the local area,” said Carrie McDonough, an assistant professor of chemistry. “There were a lot of concerns about the air quality, but air quality will get better over time. But what about the soil? And what about the surface water?”
Eleven of the railcars in the Norfolk Southern freight train derailment contained hazardous materials including vinyl chloride, isobutylene and n-butyl acrylate. Several railcars ruptured, spilling more than 100,000 gallons of the toxic chemicals. According to the Environmental Protection Agency, an estimated 8.6 million gallons of liquid wastewater has been hauled out of East Palestine. But groundwater contamination remains a concern.
To further complicate matters, three days after the train accident, crews drained the liquid vinyl chloride into a trench and conducted a “controlled burn” to prevent a deadly explosion. The fire provided the right conditions to create dioxins, highly toxic compounds that can cause cancer and reproductive, developmental and immune system problems.
McDonough, who teaches a class on Environmental Exposure and Risk Assessment, set about using the East Palestine incident as a case study. She provided students with data on the concentration of three chemicals that could have been released into sediment and water at five sites in and around East Palestine. The students used the information to determine how much the chemicals could accumulate in crayfish and small mouth bass to determine if the crayfish or fish were safe to eat.
“The fish aren’t just exposed to the chemicals from the spill. It’s also all the chemicals that are already in the water from everything that happens daily to cause exposure. It’s level after level of these calculations,” said Morgan Van Der Linde, a sophomore chemistry major. “There are so many variables that it’s definitely hard to work out.”
Classmate sophomore chemistry major Camden Johnson agreed.
“The case study was so overwhelming because you have to make all these assumptions along the way about where the chemicals can go and what they’re going to transform into,” Johnson said.
When Van Der Linde and Johnson saw an EPA fact sheet with citations from the early 2000s, they were surprised at how young the field is.
“I can see that there is a lot of progress to be made. It instilled in me confidence in the fact that I care about this, and I want to pursue this career path,” Johnson said. “I’m grateful for this program we have that is addressing these issues in a way that’s relatable to what’s happening in the real world.”
Testing for contaminants and analyzing the results will be ongoing in East Palestine for some time. The work is not straightforward, said Ryan Sullivan, a professor of chemistry and mechanical engineering, and associate director of the Institute for Green Science.
“If you don’t deal with the contamination in soils and sediments, then whatever is there can slowly leach back out over years, potentially,” Sullivan said.
Like McDonough, appreciating the complex feedback loop among soil, water and air is something Sullivan teaches students. In his Environmental Chemistry class, students use chemical principles, including structure-activity relationships, to understand how a chemical behaves in a complex environment and what its ultimate fate may be.
“For understanding a problem like East Palestine, you need a systems-level approach. It’s not just thinking about what do these molecules do. It’s what do these molecules, and these other molecules, and the water and the atmosphere and the soil and the people do — the whole system,” said Sullivan, who plans to present the train derailment as a case study.
“We know the things to be concerned about,” said Neil Donahue, the Thomas Lord University Professor in Chemistry and the director of the Steinbrenner Institute for Environmental Education and Research. “But if you don’t look with the right glasses, you won’t see them.”
Donahue, McDonough and Sullivan argue that a non-targeted analysis is key to avoid missing a possible contaminant.
“If you take a targeted approach to testing, you’re only going to find the things that you’re looking for,” Sullivan said.
McDonough uses non-targeted analysis to look for per- and polyfluoroalkyl substances (PFASs) and other organic contaminants in water, soil and humans. Sullivan uses a similar approach to look for PFASs in the atmosphere.
PFASs, a highly toxic class of chemicals, are widely used in products ranging from nonstick cookware to firefighting foam. PFAS exposure can cause cancer and developmental, endocrine, renal and metabolic problems.
“Understanding the full extent of human exposure to PFASs requires innovative analytical methodologies, including high-resolution mass spectrometry and non-target analysis,” McDonough said.
McDonough learned this firsthand while analyzing PFASs in contaminated drinking water wells near Peterson Air Force Base, Colorado. Using high-resolution mass spectrometry techniques to analyze the blood of residents, she found, in addition to the expected PFASs, a new unsaturated perfluoro-octane sulfonate PFAS in human blood.
“Studies like this highlight how much we really do need advanced analytical techniques to do this kind of work,” she said. “And to really understand the human burden, you need to look outside of traditional analytical methods.”
It is unclear whether PFAS-containing foams, which are being phased out, were used in East Palestine, but it’s a concern.
“We know that PFASs are never going to break down,” McDonough said. “A lot of the persistent chemicals that we used to use, like DDT, are still there. So we have these irreversible kinds of legacies that we leave behind. We could be doing the same thing here.”
■ Amy Pavlak Laird
Faculty in the Department of Chemistry are international leaders in the environmental chemistry of and human exposure to “everyday–everywhere” chemicals, the development of sustainable approaches to remove persistent toxic contaminants, and the study of air pollutants that create particulate matter and affect human health, clouds, and climate change. Thanks to undergraduate research opportunities and innovative courses in environmental chemistry, students leave Carnegie Mellon well prepared to tackle environmental and sustainability challenges.
Related articles

All Eyes on "Forever Chemicals"

New air quality data from East Palestine, Ohio
Exosome Tech licensed to Coya Therapeutics
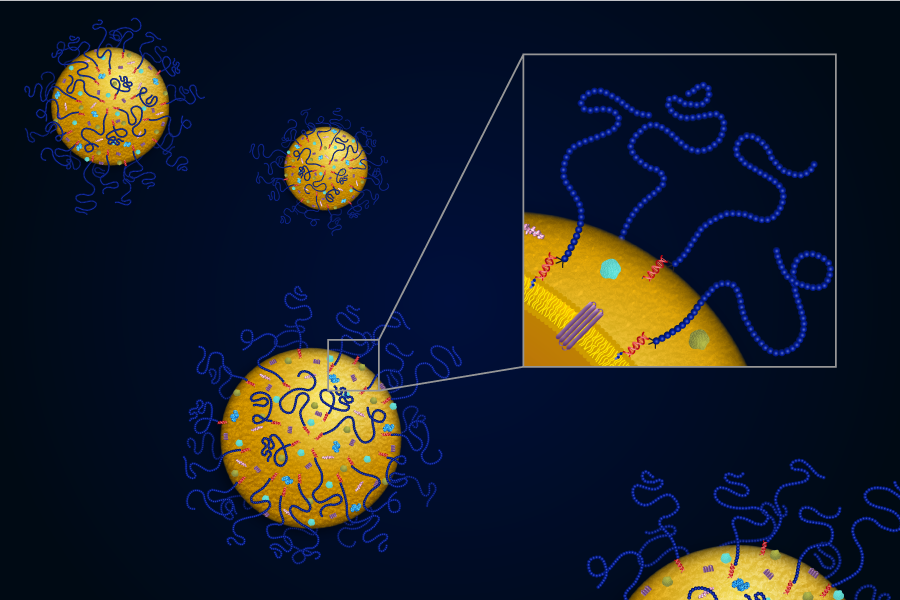
Carnegie Mellon University has licensed a proprietary platform for bioengineering exosomes for drug delivery to Coya Therapeutics, Inc. Through the agreement, Coya has optioned the exclusive worldwide rights to research, develop, manufacture and commercialize exosome polymer hybrids using the platform developed by an interdisciplinary team of Carnegie Mellon chemists and engineers.
Exosomes are a nanometer-sized type of extracellular vesicle that can be shed from every cell in our bodies and can hold a variety of cargo, including proteins, lipids, metabolites and nucleic acids. Researchers worldwide have been attempting to harness the exosome as a drug delivery vehicle, but many of the techniques that have previously been developed required the use of complex molecular biology tools or degraded the exosome’s innate functionality.
Sushil Lathwal, then a doctoral candidate in the Mellon College of Science’s Department of Chemistry, and Saigopalakrishna (Sai) Yerneni, then a doctoral candidate in the College of Engineering’s Department of Biomedical Engineering, came up with an idea to surmount these obstacles. They worked alongside researchers in the laboratories of Subha R. Das, associate professor of chemistry; Krzysztof Matyjaszewski, J.C. Warner University Professor of Natural Sciences; and Phil G. Campbell, research professor of biomedical engineering and member of the Engineering Research Accelerator, to create a method that engineers exosomes with a DNA-cholesterol tether. The synthetic single-stranded DNA on the tether can bind with a complementary strand of DNA linked to a bioactive agent.
“Our research on exosome surface modification started, in the best traditions of Carnegie Mellon University, as an interdisciplinary collaborative project. Although this project began out of simple curiosity of combining our respective research interests, we are delighted to see its potential clinical translation with our venture with Coya Therapeutics,” Das and Campbell said in a statement.
Coya Therapeutics is a clinical-stage biotechnology company that develops approaches that enhance regulatory T cell (Tregs) in vivo, including autologous and allogenic Treg-derived exosome therapeutic and novel biologics. They plan to develop exosome-polymer hybrids (EPHs) to enable exosomes to be homed to proteins of interest while delivering select payloads to targeted cells.
“This collaboration with Carnegie Mellon University further solidifies Coya’s thought leadership in the global exosome therapeutics field, adding new and exciting technologies to our unique Treg-derived exosomes platform,” stated Howard Berman, CEO of Coya Therapeutics.
“Nanoengineering exosomes with such manufacturing efficiency to produce EPHs that can be customized to any surface protein, delivering growth factors or drugs, while enhancing cellular uptake and bioactivity is the future of targeted therapies.”
“The next step of our development program will be to leverage EPHs to validate functional activity of our Treg-derived exosomes that home in and bind to high profile protein targets that drive specific disease processes. Additionally, these EPHs will be loaded with targeted payloads to enhance efficacy,” stated Adrian Hepner, president and chief medical officer of Coya Therapeutics.
■ Jocelyn Duffy
Related articles
Carnegie Mellon Researchers Create Biohybrid Nanoparticles that Could Revolutionize Drug Delivery

